|
Gas phase synthesis of biomolecules |

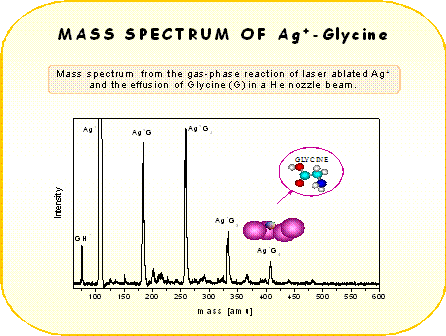
|
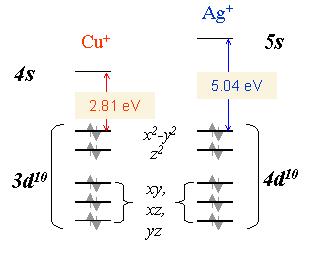
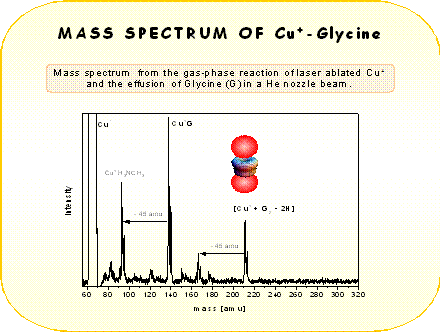
The different coordination between Cu+ and Ag+ with glycine, evident from the mass spectra,resembles the different binding behavior observed with several other molecules [3]. According to ab-initio calculations [4] copper cation tends to bind strongly two ligand molecules. This is probably due to the 4s-3ds hybridization of Cu+ orbitals and also to ligand-ligand repulsion. This mechanism reduces the electron density along the z-axis (ligand-ligand axis) allowing the closer approach of the two ligands to the Cu+ core. In contrast silver can attract up to four ligands. In the case of Ag+ the hybridization is much weaker and therefore this ion behaves like a closed shell alkali ion, where the coordination is dictated from the geometrical size of the ion. The size of Ag+ is larger than Cu+ and the ligand-ligand repulsion is reduced allowing the coordination of more ligands in the case of Ag+ .
|
|
References: M. Massaouti, M. Velegrakis, “Gas-phase Cu+– and Ag+–amino acid complexes produced with a new source”, Intern. J. of Mass Spectrom. 225 (2003) 89 |
|
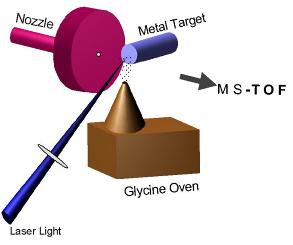
METAL ION-GLYCINE SOURCE
|
|
Metal ions (Cu+ or Ag+) are produced through the vaporization laser ablation (1064 nm) of a high purity (> 99.95 %) copper or silver rods. The plasma formed during ablation is mixed with the glycine evaporated from an effusion oven placed below the ablation point. The final complexes are “pushed” towards the spectrometer by a pulsed beam of He gas (1 bar) from a home-built nozzle. The effusion oven is simply a copper block where glycine is placed in a hole and is covered with a conical cap. The whole block is heated with the aid of a 250W halogen lamp to a temperature of 120 OC.
|